- Pharma IT consulting
- Pharma digital transformation
- Custom pharma software development
- Pharma data analytics and BI
- ERP and CRM implementation
- Business process automation
- Legacy software modernization
- Pharma software migration
- Pharma cloud computing
- Pharma mobile app development
- Pharma IT security
- CTO as a service
- 3D anatomy model development
- 3D video production
- IT outsourcing
Pharma IT consulting
Our seasoned consultants provide strategic guidance and tech-driven solutions to improve your processes and accelerate digital transformation while guaranteeing robust cybersecurity, regulatory compliance, and efficient data management.
Pharma digital transformation
Innowise's team helps pharmaceutical companies digitally transform drug development and clinical trials, enhancing collaboration and providing data-driven insights. This leads to measurable operational efficiency improvements, keeping them ahead of the curve.
Custom pharma software development
We develop custom pharmaceutical software solutions from scratch, as well as pilot and PoC solutions. Handling every stage from requirements gathering to design, deployment, and maintenance, our experts stay responsive to your needs and make necessary adjustments.
Pharma data analytics and BI
Our company creates custom analytics and BI solutions that turn complex pharmaceutical data into actionable insights. These tools collect, integrate, and analyze data from various sources, including clinical trials, sales data, and market research.
ERP and CRM implementation
Our team excels at
customizing ERP and CRM platforms like SAP, MS Dynamics 365, and Salesforce. By streamlining R&D, production, distribution, and sales processes, we help you bring drugs to market faster and more effectively.
Business process automation
Our team assesses your R&D, clinical trials, manufacturing, and other processes to identify automation opportunities. We implement custom solutions to reduce costs, minimize errors, ensure compliance, and free up resources for strategic activities.
Legacy software modernization
Innowise assists in upgrading your legacy systems to modern, scalable pharmaceutical software solutions tailored to your evolving needs. Our experts provide a seamless transition and secure data migration, minimizing operational disruptions and maximizing the value of your software investment.
Pharma software migration
Our team expertly manages complex migration processes, seamlessly transitioning your software and data to new environments. Whether migrating to the cloud or modernizing your existing infrastructure, we minimize downtime and guarantee data integrity.
Pharma mobile app development
Innowise expert developers help businesses build cutting-edge pharmacy apps, including those for medication delivery, prescription reminders, and doctor consultations, to improve patient care and streamline operations.
Pharma IT security
Our experts are dedicated to protecting your sensitive data, intellectual property, and research findings from cyber threats. We implement comprehensive security strategies tailored to your unique requirements to ensure compliance and mitigate risks.
CTO as a service
Innowise's CTO as a service model provides pharmaceutical companies with flexible access to experienced CTOs. They help businesses improve their technology strategy and optimize operations without the cost and commitment of a full-time hire.
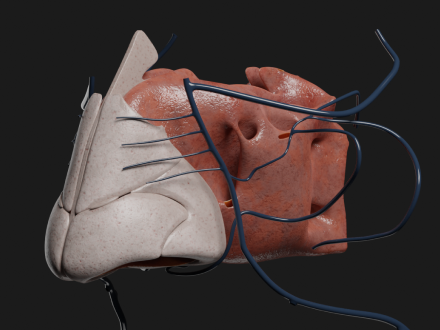
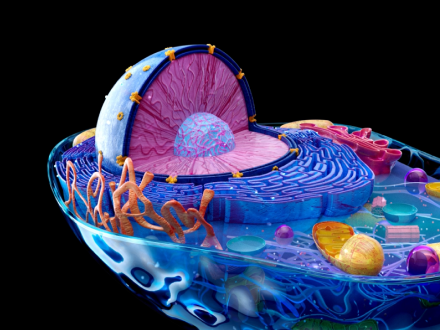
3D anatomy model development
To support pharma R&D innovation, Innowise creates highly accurate 3D models of human organs. These detailed models offer invaluable insights for research, testing, and training, thereby accelerating innovation and enhancing patient outcomes.
3D video production
Innowise produces 3D videos to educate both doctors and patients about the effects of medications on the body. These captivating videos broaden understanding and promote informed decision-making, improving patient care and treatment outcomes.
IT outsourcing
Innowise offers flexible IT outsourcing models, including turnkey product development, dedicated project teams, and staff augmentation services to refine your existing projects with our expert pharmaceutical software engineers.